It is estimated that around 5% of those infected by the coronavirus will need intensive care and the help of a respirator. This percentage depends on the number of tests and some studies question its effectiveness, but beyond the statistics, the truth is that respirators have become one of the most necessary devices in this crisis. And there are not enough to care for all the patients.
These respirators or “mechanical ventilators” save time for the treatment to take effect, ventilating the patient and oxygenating the lungs. They are machines that we can find in hospital ICUs, but their price can exceed 15,000 euros and their availability is insufficient.
The Government of Spain has requested the purchase of 4,000 respirators, but the arrival of the shipment is not easy. Given the difficulty in obtaining respirators abroad, Spanish companies and universities such as SEAT, the URJC, and the University of Malaga have started the production of artificial respirators. A race to produce new respirators in the shortest possible time, since many lives depend on them. But these projects have run into an added problem: their certification.
Who is in charge of the production of respirators?

According to the count carried out by the Spanish Society of Intensive Nursing and Coronary Units (SEEIUC), Spain had 2,487 ventilators, an average of 17 per ICU. This is not the total number, but rather the response of 59% of the ICUs in this country. And the problem is that there was no official number of respirators available in Spain.
That was at the beginning of the crisis, but the number of seriously hospitalized has exceeded the number of respirators. A problem repeated worldwide has caused the price of these respirators to double and global demand to skyrocket. A demand well above production has caused a stock out.
Along with 35 million masks and more than half a million PCR kits, Spain has requested thousands of respirators through joint purchase processes from the European Union, but it is estimated that these could take several months. And in the fight against COVID-19, time is a key factor.
The main producers in Europe of respirators are Medtronic (Ireland), Dräger (Germany), Getinge (Sweden), and Hamilton Medical (Switzerland), but their total production, estimated at around 10,000 units per year, is not enough. To compensate, the governments of the main countries have encouraged different companies to produce their emergency respirators. And what companies can produce them? Mainly car manufacturers, since they can adapt motors and components to produce mechanical fans .
This has led companies such as the FCA group and Ferrari to manufacture respirators for Italy, PSA and the manufacturers Peugeot and Citroen to manufacture up to 10,000 respirators for France, Mercedes Benz to reuse its Formula 1 technology, Ford to produce 50,000 units for the US or even Dyson’s digital motor has been adapted to meet the requirements and produce 15,000 fans.
They are not the only private companies that are collaborating in the production of respirators. The Zurich group has donated 200,000 euros to promote the development of new models of emergency respirators and manufacturers such as Medtronic have decided to release the development of their PB 560 portable respirator .
What do artificial respirators need to be approved?

With the need for respirators, many companies have launched to try to collaborate, but not all projects work. The Ministry of Health has warned that only those who comply with the necessary approval and provide the requested documentation and tests will be authorized.
This certification is important since without it these respirators do not have the requirements to be able to connect with the health system and therefore cannot be used in ICUs intended to care for patients in serious condition.
Several of the projects that are underway are based on industrial parts and components used in the manufacture of automobiles. In the case of SEAT, the windshield motor has been readapted for its mechanical respirator, although the entire device has more than 80 electronic and mechanical components.
Although it fulfills the function of helping to ventilate, we are not dealing with a state-of-the-art respirator and authorization is not trivial. The circumstance has arisen that while the companies and research groups worked against the clock to have them account before, from Health they have wanted to be sure of its correct operation. A difference in positions has led the SEAT works council to criticize the “excessive bureaucracy at a time when the important thing is to save lives”.
The Spanish Medicines Agency (AEMPS) explains that these respirators are “very precise and complex invasive equipment”. They indicate that “its design and functionality must guarantee, in addition to fulfilling its original function, that its use does not compromise the clinical status or the safety of patients, nor the safety and health of users.”
Given the numerous respirator manufacturing projects, both 3D printing and adaptations or new prototypes, the AEMPS explains that, as in the rest of the countries of the European Union, to market these devices they must be “provided with the CE marking, a hallmark that declares the conformity of the product with the safety, efficacy and quality requirements established in the legislation”.
Once these results are received, the next step is to request a clinical investigation regulated by Royal Decrees 1591/2009 on medical devices and RD 1616/2009 on active implantable medical devices.
Among the aspects that the AEMPS recalls with these artificial respirators is that it must be taken into account that, like all electromedical equipment, “mechanical fans emit electromagnetic radiation that can affect the operation of the rest of the patient’s equipment.” Equivalently, “it must be verified that the operation of the prototype is not affected by the electromagnetic emission of the rest of the usual equipment in an ICU”. The latter, the certificate on electromagnetic, was precisely what has most delayed respiratory projects such as seats.
However, due to the health crisis, the AEMPS is aware of the growing number of patients and has therefore prepared a document indicating the technical documentation and minimum tests that have to be carried out on these prototypes. Remember that “even in emergency conditions, it is only possible to use them in the context of clinical research that identifies their efficacy and safety profile”.
The first step to receiving certification is to submit detailed technical documentation, including technical specifications, layout, identification of similar equipment, a brief risk analysis, identification of safety requirements, and a description of the manufacturing and preclinical test results. These should include operational tests in human models with artificial lungs, validation results in pigs, and functional safety trials of the prototype.
The Spanish Medicines Agency works with six projects
On April 1, the Spanish Medicines Agency reported that “6 projects are being worked on at a fairly advanced stage”. Beyond these six chosen projects, others are in the earlier stages of their development but have not yet passed the requirements to continue with their essay.
To all of them, the AEMPS has offered “continuous support and technical support to speed up development and achieve safe prototypes that can be tested within clinical research protocols in hospitals.”
Some of these projects arose through a WhatsApp conversation, which led to the creation of the Innovative Breathing Assistance (AIRE) forum, as described by the Sinc Agency. The group is directed by Jorge Barrero, general director of the Cotec Foundation. As a result of the initiative, the AEMPS created the technical requirements document so that these projects know what they must comply with to be of help with their respirators.
Among the different projects, the only ones that have so far received the green light to start large-scale production are the SEAT and Hersill respirators. Beyond the two companies, these are some of the Spanish projects that are working on artificial respirators to help deal with COVID-19.
Andalusia Breathe

A group of engineers from the University of Málaga ( UMA ), together with researchers from the Biomedical Research Institute of Málaga (IBIMA) and doctors from the Regional de Málaga and Virgen de la Victoria University hospitals are responsible for the Andalucía Respira project. This is one of the artificial respirators that are in the process of being evaluated by the Spanish Medicines Agency.
Ignacio Díaz de Tuesta, a cardiovascular surgeon at the center and author of the original idea for the respirator, which he embodied in his doctoral thesis, explains together with the General Secretary for Research, Development and Innovation of the Ministry of Health and Families, Isaac Túnez, that They hope that their respirator can be produced within seven to ten days, “once a second clinical trial on a real patient is successfully carried out and the latest technical tests are passed.”
In a statement on April 3, the group reported that this second human trial had been completed. Specifically, “the prototype of this device has been tested on a patient with Covid-19 who is in the ICU of this Andalusian public health hospital” explained the IBIMA researchers.
Initial tests include a trial with a patient admitted to the ICU of the Hospital de Antequera and a trial on an artificial lung. It was also experimented on in an animal model and on April 2 in a patient with acute respiratory failure in the ICU of the Virgen de las Nieves Hospital in Granada.
The prototype has a two-hour run time and has no moving components. The electrical controller is based on commercial automata and has been certified by the AEMPS after sending the technical documentation. Among the tests that remain to be passed to be officially applied are the electromagnetism certificate and a test of the autonomy of the device with UPS, which must last 48 hours.
The Andalucía Respira team has already received several proposals for its preparation from companies in Cádiz, Jaén, Málaga, and Seville, which could each launch an average of 50 respirators a week. One of them is Fujitsu, which is already adapting its production line in Malaga.
Resistance Team

Another of the projects that is pending validation from the Ministry of Health is that of the Resistencia Team, within the group of ‘Coronavirus Makers’. Their work is based on the Jackson Rees open source system and these days they have received the collaboration of the Renault company to start the production of their respirators through 3D printers. Help that does not only reach the manufacturing level, but the company group itself has proposed several improvements in the prototype valve.
With the coordination of the Ministry of Science and the University of Oviedo, these days the respirator prototype is being clinically tested at the Central University Hospital of Asturias (HUCA).
Leitat
The Leitat1 project is carrying out clinical and safety tests this weekend, with the expectation of trying to implement it in five days. The Catalan company Leitat has collaborated on the project, together with the Barcelona Free Zone Consortium, HP Spain, Almirall, and the Terrasa Health Consortium. It is a field respirator, not a UCI one, of which they assure that they will be able to produce between 50 and 100 units per day if they receive the necessary approval.
Initially, the prototype, made with 3D printers, was designed to intubate a patient for three or four days but no more because the air pressure was not adjustable. But in the latest models, volumetric and pressure sensors and oxygen alarms have been added to improve your safety.
The Open Ventilator
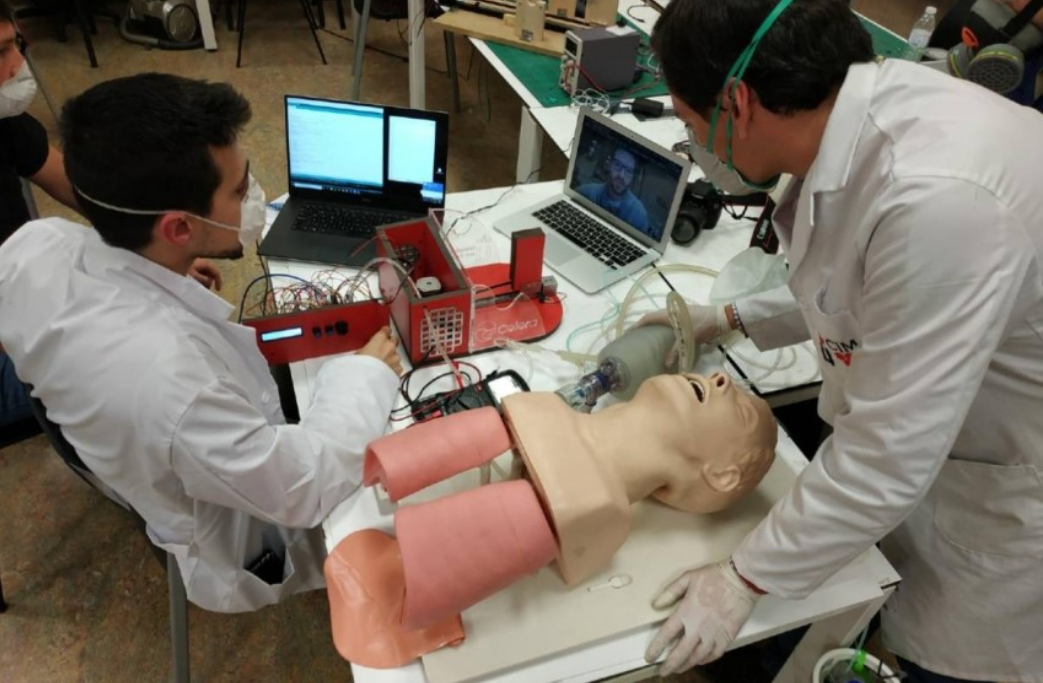
It is a project devised by Javier González, a materials engineer from the Rey Juan Carlos University ( URJC ). The Open Ventilator is a joint work of the Faculty of Health Sciences.
As the creators describe, “The Open Ventilator can be mass-produced with the tools commonly found in any mechanical workshop, it doesn’t even require 3D printing. All the components used also have European certification.” It is an open prototype and has been promoted by Celera, a national network of young talent. These days, the Official Central Electrotechnical Laboratory has granted them the approval to continue with their production.
Acute-19
The Acute-10 project is presented by Oximesa Nippon Gases together with the Faculty of Veterinary Medicine of the CEU Cardenal Herrera University and the La Fe University and Polytechnic Hospital in Valencia. It is a turbine respirator and on April 3 they reported that they had completed phase 3, after testing without patients in a clinical simulation station and testing the prototype on animals.
“The Acute-19 turbine ventilator demonstrates its capabilities to maintain ventilation in a severe respiratory distress model, also allowing weaning and supporting the ventilation of the patient with CPAP.
The turbine respirator will be available in open source and it is expected that its mechanization for production could begin in about 15 days.
Other projects
Other projects that are having more difficulties in receiving approval from the Spanish Medicines Agency are the project of the Catalan company Noel, with electronic control for air inspiration, and the project of the Aragonese engineer Jorge Cubeles, who has developed a respirator with an internet connection. An addition that would enable the status of patients to be analyzed from a remote control post.
However, these electronic additions have meant an added difficulty when it comes to obtaining their approval, despite having the support of companies such as Balay, willing to offer their machinery. And it is that of the 40 proposed respirator projects, only six are still under evaluation and only two already have full approval to start production.
SEAT starts production of its artificial respirator: up to 300 units per day

The largest artificial respirator project is OxyGEN, the result of the joint work of Protofy.XYZ, SEAT, the Germans Trias I Pujol Hospital and Research Institute, the Hospital Clínic, and other companies and administrations such as the University of Barcelona, Recam Laser, Doga Motors, Luz Negra, LCOE, Ficosa, Bosch, IDNEO, Secartys, Cuatrecasas, Spiroflex, Gaso and the National Police, Civil Guard, Urbana, and Mossos d’Esquadra. As we can see, a project that has brought together multiple organizations and teams to achieve an approved and functional artificial respirator.
The Spanish Medicines Agency has chosen OxyGEN as one of the two respirators that can already be produced on a large scale to be applied in hospitals. As announced by the Minister of Health, Salvador Illa, SEAT will produce up to 300 emergency respirators per day on the SEAT León assembly line in Martorell over the next few days.
The last step to achieve certification has been to obtain the device’s static magnetic electricity test to ensure that it does not interfere with the rest of the UCI equipment. During these days, the SEAT teams have worked against the clock to pass the required tests.
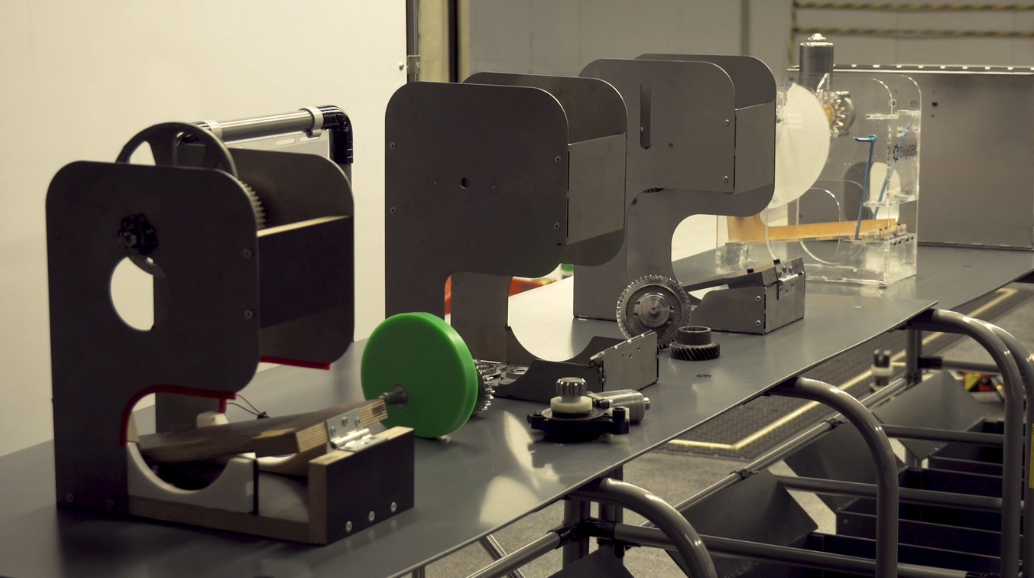
SEAT has produced these assisted respirators by adapting a wiper motor. A total of 150 employees from different areas began working a week ago on the development of this prototype, after 13 different models .
“Each respirator has more than 80 electronic and mechanical components and passes an exhaustive quality control with ultraviolet light sterilization,” explains the company. The device has been designed by the free hardware company Protofy. XYZ under the medical direction of doctors Dr. Manel Puig Domingo (Germans Trias I Pujol Research Institute), Dr. Oriol Estrada (Germans Trias I Pujol Hospital), and Dr. Josep María Nicolás (Clínic Hospital).
Among the parameters that are measured are heart rate, blood pressure, body temperature, volume and pressure of inspiratory air that the ventilator provides, oxygen saturation, and CO2 of exhaled air. Two of these respirators are already in Can Ruti and can be used after receiving permission from the Spanish Medicines Agency. From SEAT they directly thank the director of the agency and the Minister of Health himself for their involvement. After momentarily halting production due to the uncertainty of its certification, SEAT will finally be able to manufacture this respirator on a large scale, adapt its production lines, adapt shifts, and help health services.
Hersill and its VIATE 40: the largest national producer receives the order from the Government

Located in Móstoles, founded in 1987 and with 60 workers, the Madrid SME Hersill was already the largest producer of respirators nationwide. But its production level was below what this crisis needs.
Your VITAE 40 lung ventilator weighs less than 1.4 kg and is designed to be held in one hand. The emergency ventilator includes “all modes of ventilation, integrates capnography (including an interface for volumetric capnography) and wireless communications (Bluetooth and Wi-Fi) options for selective downloading of ventilation records for real-time or remote analysis.” from the hospital. It also includes specific ventilation modes for CPR and exclusive assistants (CPR wizards) according to the latest ERC and AHA guidelines.” A complete and approved respirator that has received an important boost to deal with COVID-19.
Military engineering company Escribano Mechanical & Engineering has teamed up with Hersill to produce up to 5,000 lung ventilators over the next 8 weeks. A request that comes directly from the General Secretariat of Industry.

Last Friday, the Prime Minister himself, Pedro Sánchez, together with the Minister of Health, Salvador Illa, visited the Hersill facilities to observe first-hand this initiative to manufacture respirators. In total, Escribano and Hersill aim to produce about 100 respirators a day. That is, multiply by ten the production of 10 units per day that Hersill maintained to date.
If the plans continue as planned, Spain will be able to supply itself with respirators in June and it could even be possible to create a surplus. Some devices could be exported to those countries that are in a situation similar to the one that now affects Spain.
Sharlene Meriel is an avid gamer with a knack for technology. He has been writing about the latest technologies for the past 5 years. His contribution in technology journalism has been noteworthy. He is also a day trader with interest in the Forex market.











